
Potential Energy Kinetic Energy And Total Energy Of The Electron In Bohr S Orbit For Jee And Neet Youtube

1 Uu D 0 07 D 1 If The Kinetic Energy Of Electron Moving In 4th Orbit Of Hydrogen Is Then The Total Energy In 1 Orbit Of Li2 Is A 144
Using Bohr S Postulates Obtain The Expression For I Kinetic Energy And Ii Potential Energy Of The Electron In Stationary State Of Hydrogen Atom Sarthaks Econnect Largest Online Education Community

If We Assume Only Gravitational Attraction Between Proton Mass M And Electron Mass M In A Hydrogen Atom And Also Assume Bohr S Quantization Condition Then The Expression For The Nth Orbit Energy

In Bohr S Orbit Kinetic Energy Of An Electron In The N Th Orbit Of An Atom In Terms Of Angular Momentum Is Propotional To

Potential Energy Kinetic Energy And Total Energy Of The Electron In Bohr S Orbit For Jee And Neet Youtube

Calculate The Energy Of An Electron In Second Orbit Of An Excited Hydrogen Atom The Energy Of Th Youtube

The Mangnitude Of Pontential Energy Of Electron In N Th Excited State Of He Ion Is 8 81 Time The Kinetic Energy Of Electron Of Excited State Of Li 2 Ion Find N

Total Energy Of An Electron In First Orbit Of Hydrogen Atom Is 13 6 Ev The Kinetic Energy In The Same Orbit Will Be
In A Hydrogen Atom The Kinetic Energy Of An Electron Is 3 4ev What Is The Distance Of That Electron From The Nucleus Quora
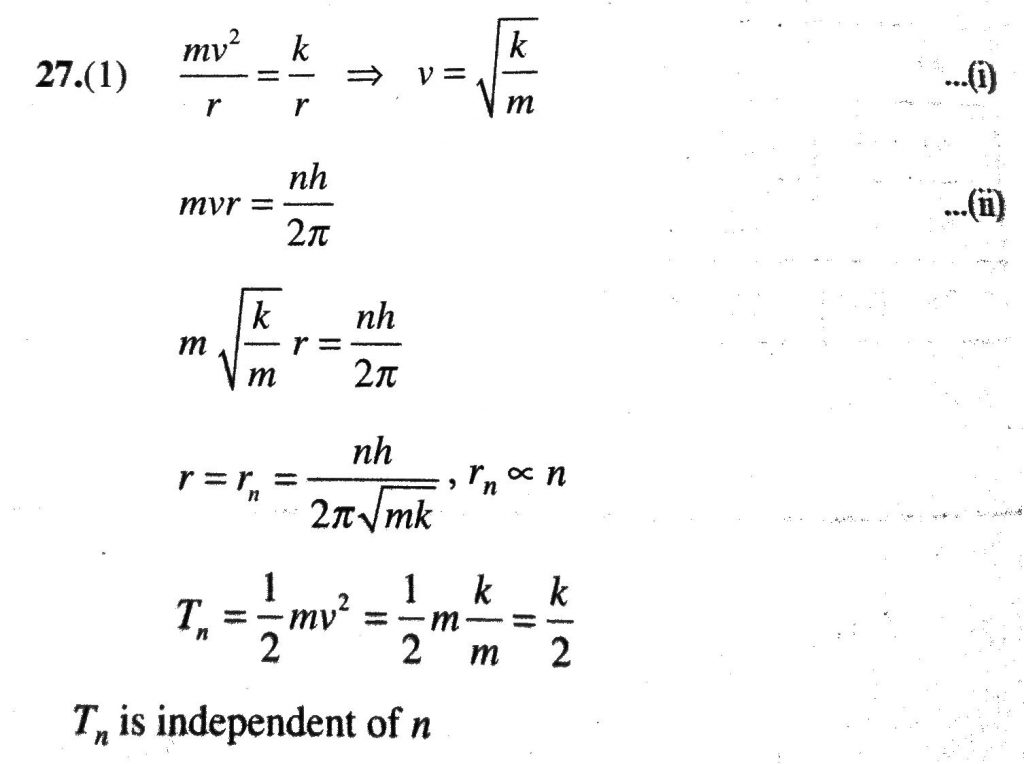
Suppose An Electron Is Attracted Toward The Origin By A Force K R Where K Is A Constant And R Is The Distance Of The Electron From The Origin By Applying Bohr Model

Kinetic Energy Of Electron In A Mono Electronic Species Is 1312kj Mole Then Which Of The Following Statements Are Correct 1 The Electron Is Present In The 2nd Orbit Of He Ion Ii

The Total Energy Of An Electron In The First Excited State Of The Hydrogen Atom Is About 3 4 Ev A What Is The Kinetic Energy Of The Electron In This








No comments:
Post a Comment