We address the electronic structure of a twisted two-layer graphene system showing that in its continuum Dirac model the moiré pattern periodicity leads to moiré Bloch bands. A moiré pattern is formed when two copies of a periodic pattern are overlaid with a relative twist.

Periodic Table Photographic Print Pasieka Allposters Com In 2021 Periodic Table Element Quiz Periodic Table Of The Elements

Carbon Atomic Structure Stock Image C018 3687 Science Photo Library Atomic Structure Atom Electron Configuration
This is because such an atom has only a single valence electron.

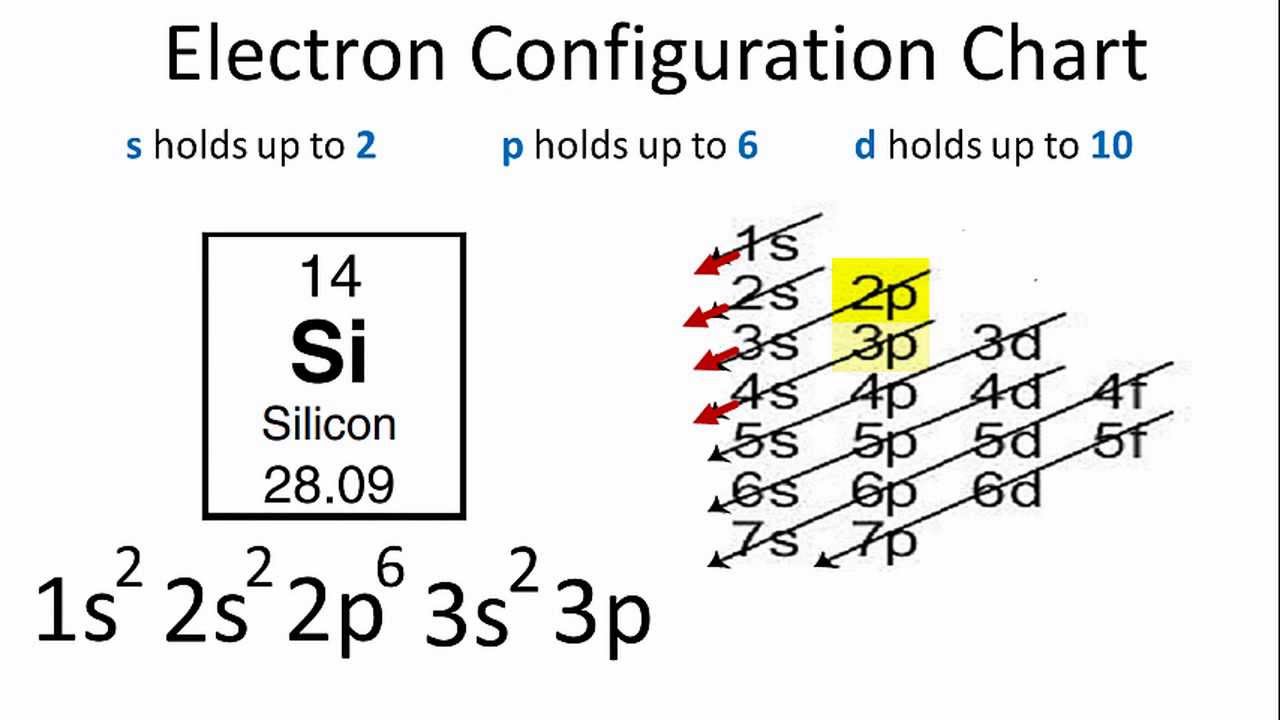
How many valence electrons does si have.
On the Periodic Table of Elements elements are arranged in columns known as groups and rows known as periods Groups contain elements with similar properties that have the same electron arrangement in their outer shells known as valence electrons which determine the properties of the element and its chemical reactivity and how it will take part in chemical bonding.
Write the complete electron configuration for each isotope.
Non-metals are those which lack all the metallic attributes.
1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 5.
When numerous phosphorus atoms are substituted for silicon in a crystal many free electrons become available.
Therefore elements whose atoms can have the same number of valence electrons are grouped together in the periodic table of the elements.
The charge on the anion is the group number minus eight.
I has 53 protons 53 electrons and 78 neutrons.
Co has 27 protons 27 electrons and 33 neutrons.
Non-metals usually have 4 5 6 or 7 electrons in their outermost shell.
The two layers become more strongly coupled and the Dirac velocity crosses zero several times as the twist angle is.
3s 3p 3d 2 6 10 18 however elements in the 3rd period only have up to 8 valence electrons.
Heterostructures in which electrons and holes have their lowest energies in different materials are called Type II but such structures will not be considered further here.
There are 3 types of electrons.
They are good insulators of heat and electricity.
Bound electrons are the inner shells electrons that are under strong Coulomb forces from nucleus and difficult to detach.
Main-Group Nonmetals Groups IVA VA VIA and VIIA.
Since the 1980s the more difficult problems of implementing valence bond theory into computer programs have been solved largely and valence bond theory has seen a resurgence.
The anion is named by taking the element stem name and adding the ending -ide.
Valency of Nitrogen by Richard-January 28 2021 0.
The electron configuration for the first 10 elements.
The magnitudes of F 13 and F 23 are all in SI units.
Theres space for 18 texte- in the 3rd shell.
Nitrogen a chemical element with the symbol Si and atomic number 14 is a colorless liquid gas or solid.
The number of valence electrons in an atom governs its bonding behavior.
Bound electrons valence electrons and free electrons.
Ions of Some Nonmetals Groups IVA - VIIA.
H 1s1 He 1s2 Li 1s2 2s1 Be 1s2 2s2 B 1s2 2s2 2p1 C 1s2 2s2 2p2 N 1s2 2s2 2p3 O 1s2 2s2 2p4 F 1s2 2s2 2p5.
At normal temperature and pressure two atoms of nitrogen bind together to form colorless and odorless dinitrogen N 2 gas.
Four of its valence electrons take over the bonding responsibilities of the four silicon valence electrons that they replaced.
How Many Valence Electrons Does Nitrogen N Have.
Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table.
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 7.
Group IVA VA VIA and VIIA nonmetals tend to form anions by gaining enough electrons to fill their valence shell with eight electrons.
Some donors have fewer valence electrons than the host such as alkali metals which are donors in most solids.
The most reactive kind of metallic element is an alkali metal of group 1 eg sodium or potassium.
A p-type p for positive semiconductor is created by adding a certain type of atom to the semiconductor in order to increase the number of free charge carriers.
In this particular material system both electrons and holes see higher energies in the AlGaAs than in the GaAs giving a so-called Type I system.
The highest occupied electron shell is called the valence shell and the electrons occupying this shell are called valence electrons.
The total number of electrons present in the valence shell of an atom are called valence electrons and there are a total of four electrons present in the valence shell of carbon 2s 2 2p 2.
Valency of Carbon C.
Theres an important distinction between the number of electrons possible in a shell and the number of valence electrons possible for a period of elements.
But the fifth valence electron remains free without bonding responsibilities.
When the doping material is added it takes.
How many protons neutrons and electrons are in atoms of these isotopes.
In the third period of the table the atoms all have a neon-like core of 10 electrons and shell 3 is occupied progressively with eight electrons starting with the 3s-orbital.
F 13 kq 1 q 3 r 13 2 90E9.
Non-metals are the elements which form negative ions by accepting or gaining electrons.
Thus carbon has four valence electrons.
The impact of valence theory declined during the 1960s and 1970s as molecular orbital theory grew in usefulness as it was implemented in large digital computer programs.

Print This Handy Periodic Table With Valence Charges Periodic Table Printable Periodic Table Of The Elements Periodic Table Chart

Science Education Trends In Atomic Radius In The Periodic Table Teaching Chemistry Chemistry Classroom Chemistry Lessons

Electronic Configurations Chemwiki Electron Configuration Complex Sentences Worksheets Text Features Worksheet

Science Coverage Valency Of Cesium How Many Valence Electrons Doe In 2021 Electron Configuration Electrons Noble Gas

Science Coverage Is Clo3 Polar Or Nonpolar In 2021 Molecular Geometry Covalent Bonding Oxidation State

Students Will Be Able To Identify Valence Electrons Determine Ions Formed And Explain The R Physical Science Lessons Chemistry Worksheets Chemistry Classroom

Oxygen Electron Configuration How To Write The Electron Configuration For Oxygen O In 2021 Electron Configuration Electrons Oxygen

Valence Electrons Coloring Activity Chemistry Science Pdf Printable From Laurelsusanstudio On Teache Chemistry Activities Chemistry Classroom Science Chemistry

Dynamic Periodic Table Hd Images Dynamicperiodictable Periodic Table Periodic Table Of The Elements Teaching Chemistry
Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcqgtf59q5s9i9gngbwkbd60z4tqcl Ckgqjpu0wd8l1itpdlpr3 Usqp Cau

Oxygen Electron Configuration How To Write The Electron Configuration For Oxygen O In 2021 Electron Configuration Electrons Oxygen












No comments:
Post a Comment