

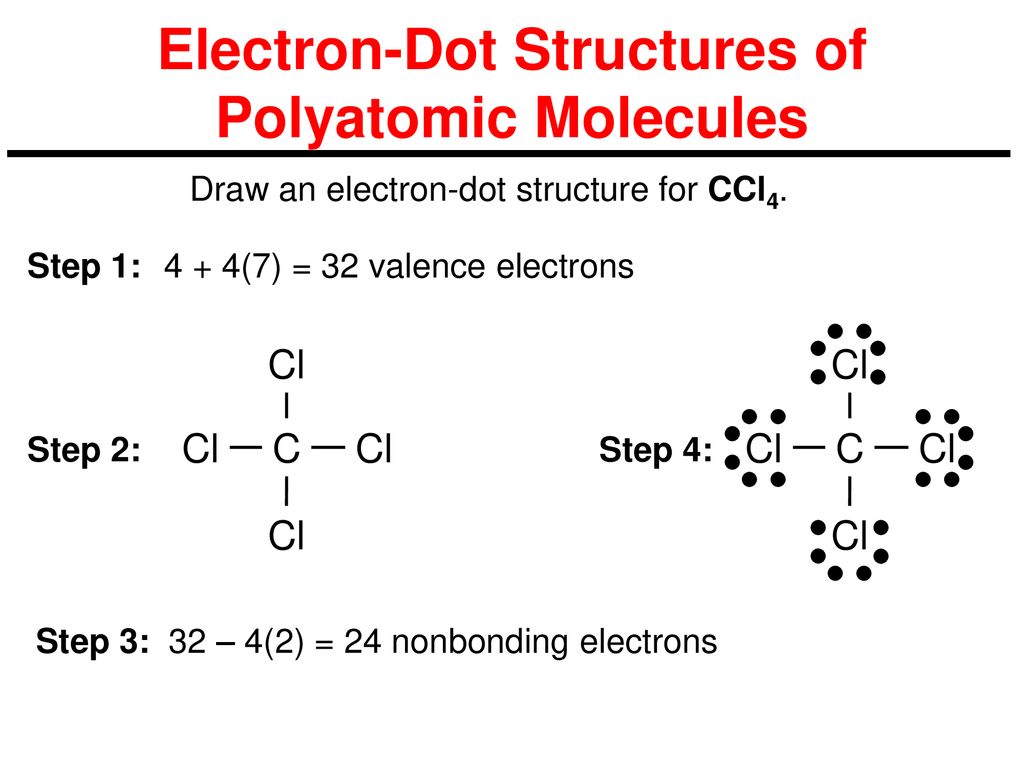
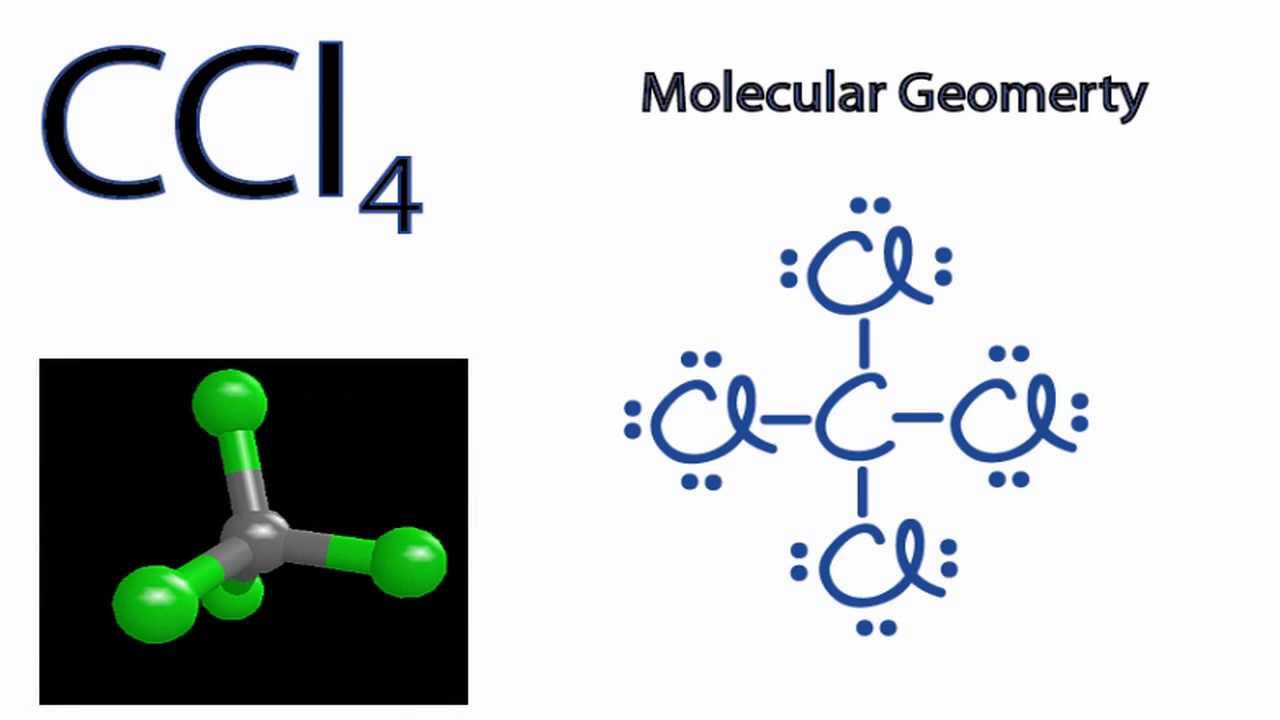
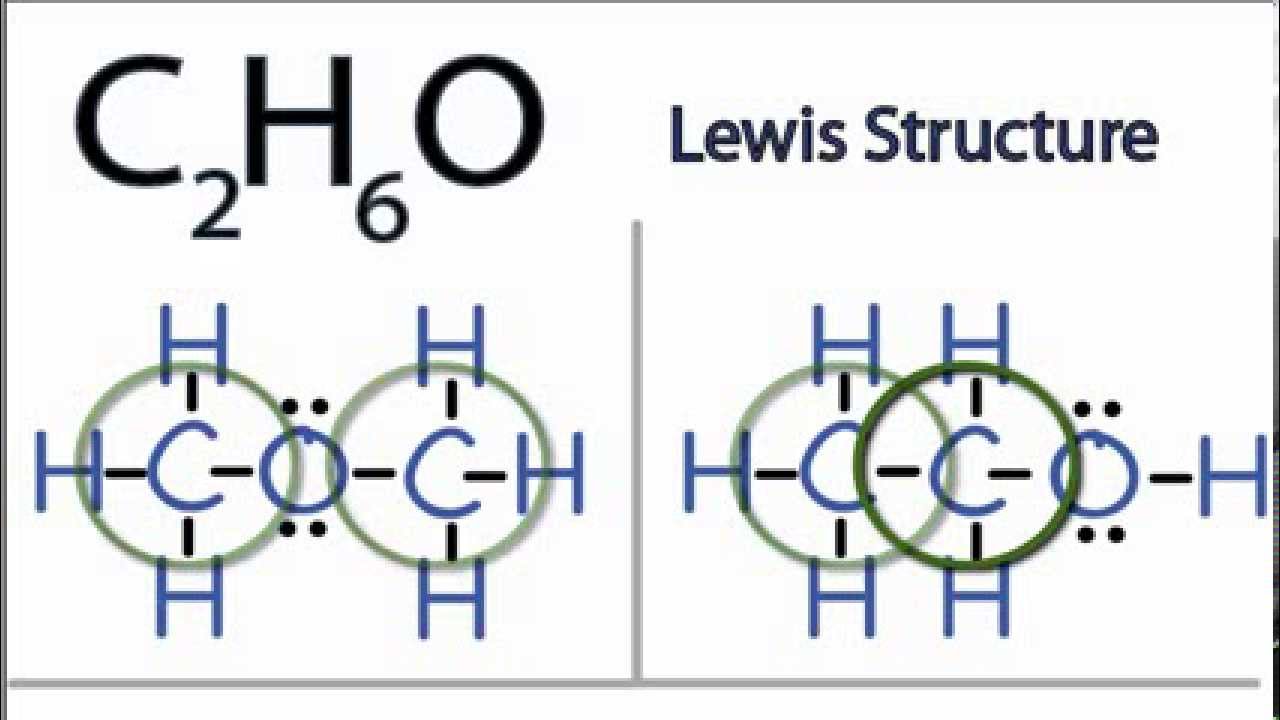
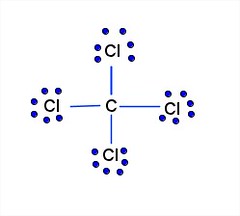
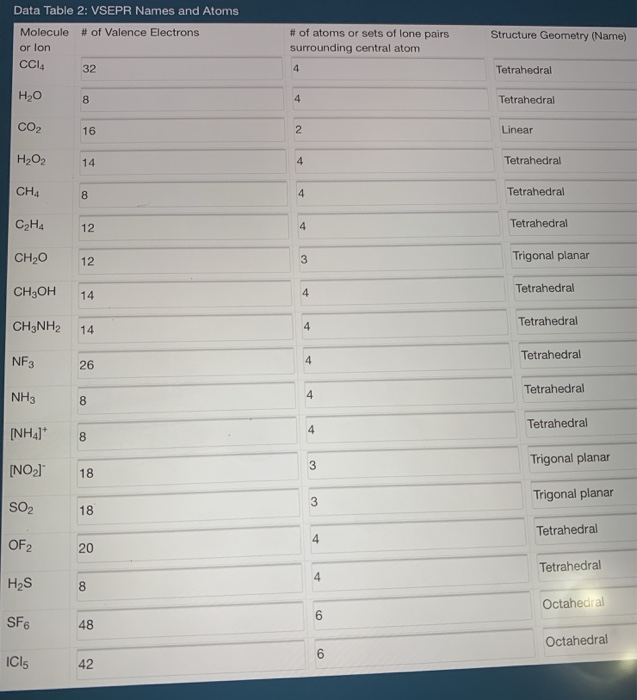
How Many Pairs Of Electrons Are Shared By The Carbon Atom With Each Chlorine Atom In The Formation Of Carbon Tetrachloride Enotes Com

Construct The Lewis Structure Model For The Covalent Compound Carbon Tetrachloride Ccl4 Using The Following Steps 1 The Total Number Of Valence Electrons In Ccl4 Is 2 Write The Atomic Core












No comments:
Post a Comment