Four main bonding types are discussed here. How does ferritin store iron.
4 of the 5 orbitals will have one electron and 1 orbital will have two electrons.
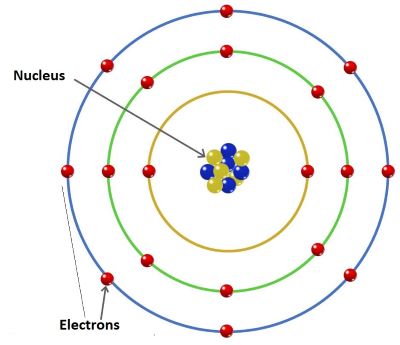
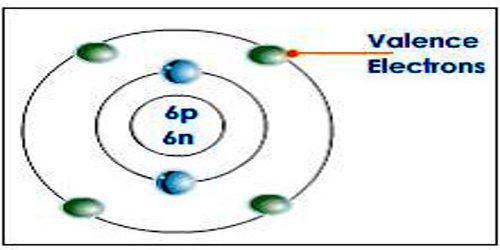
How many valence electrons does iron have.
Crystal Field Theory How many unpaired electrons are there in a strong field ironII octahedral complex.
ˈ r ɛ d ɒ k s RED-oks or ˈ r iː d ɒ k s REE-doks is a type of chemical reaction in which the oxidation states of atoms are changed.
According to Paulis Exclusion Principle.
The single electrons in the 3d level are NOT involved in the bonding in any way.
Iron has the electronic structure.
The alkali metals lithium and sodium each have only one valence electron the alkaline earth metals beryllium and magnesium each have two and the halogens fluorine and.
The d electron count is a chemistry formalism used to describe the electron configuration of the valence electrons of a transition metal center in a coordination complex.
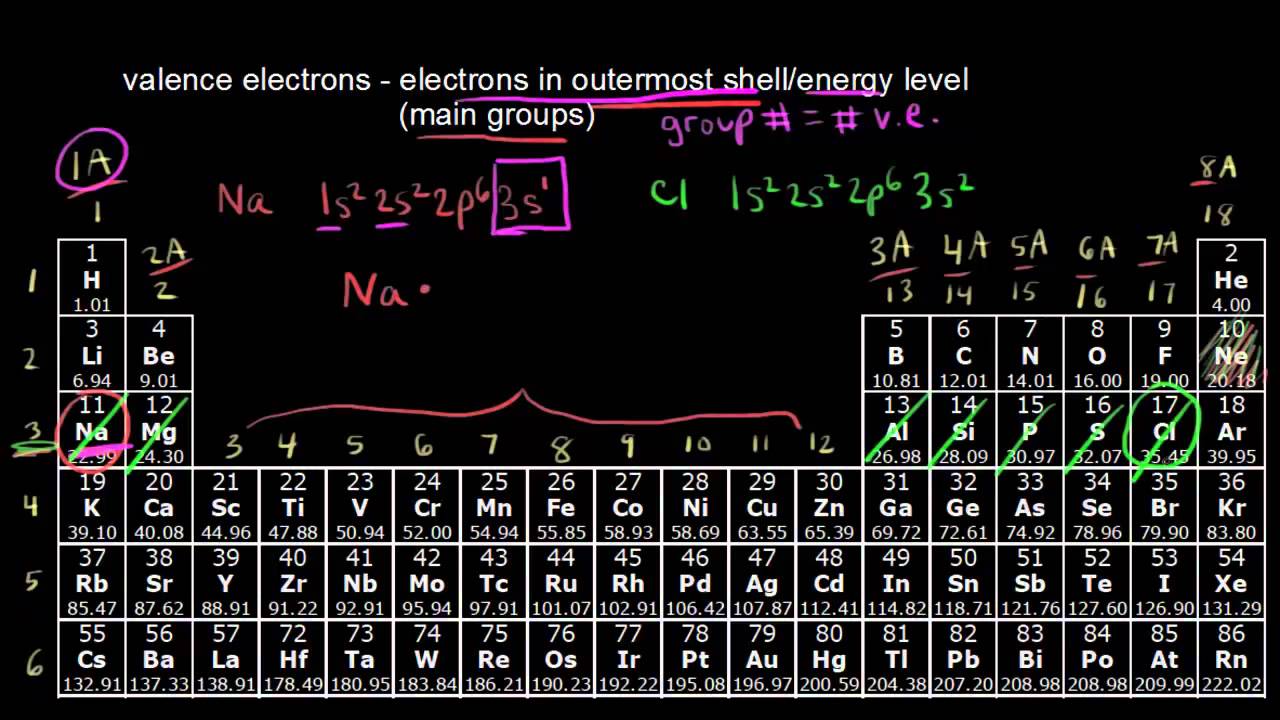
There are 7 valence electrons because the highest energy level 3 has 7 total electrons 5 plus 2 is 7.
For calcium which has an atomic number of 20 and therefore 20 electrons find calcium on.
The simplest and most important rule for predicting how atoms form compounds is that atoms.
How Many Valence Electrons Does Calcium Ca Have.
Valency of Calcium by Richard-January 31 2021 0.
Iron is in group 8 so.
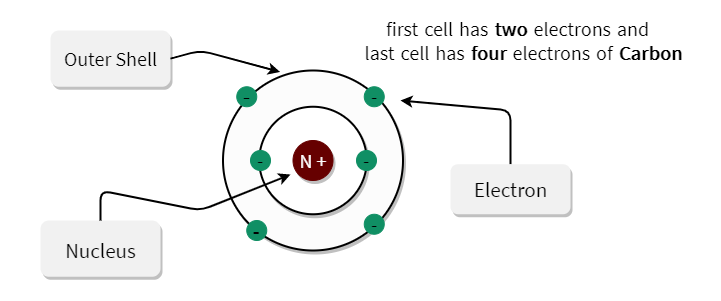
The outer shell is called the valence shell.
The higher the number of valent electrons the more reactive the atom or molecule is.
1s 2 2s 2 2p 6 3s 2 3p 6 3d 5.
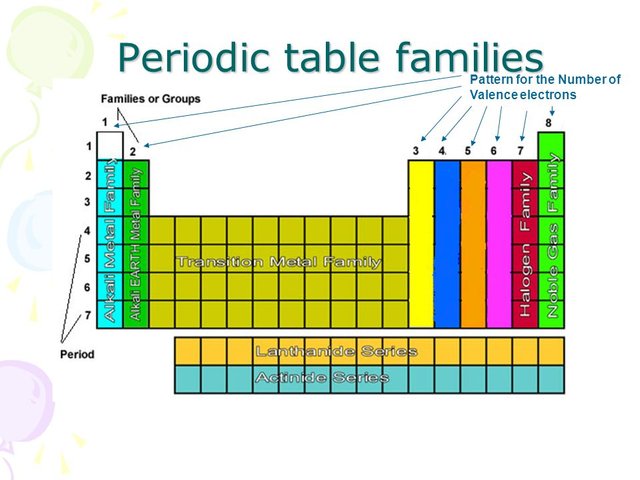
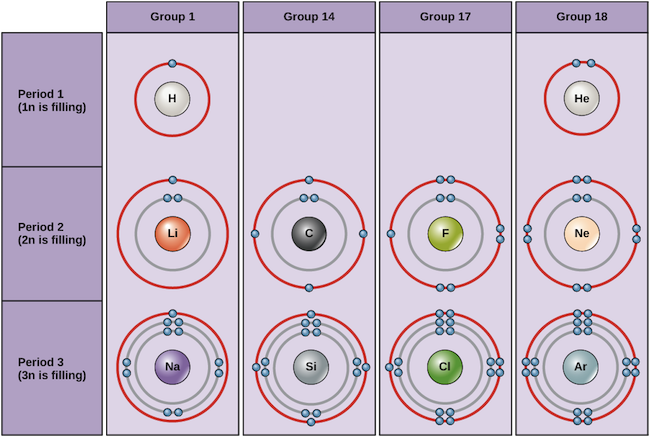
Elements in any one group or column have the same number of valence electrons.
Crystal - Crystal - Types of bonds.
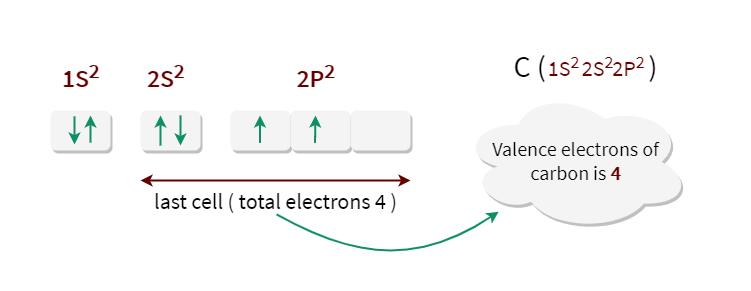
Thus a carbon ion can have a charge of anywhere from -4 to 4 depending on if it loses or gains electrons.
Ionic covalent metallic and molecular.
Gold has 79 protons in each atom and has an atomic number of 79.
This is the number of protons in each atom.
Phosphorus can even form the PF 6-ion in which there are 12 valence electrons on the central atom as shown in the figure below.
The orbital diagram looks like.
There are many examples of solids that have a single.
Hydrogen is the first element and has one proton so it has an atomic number of 1.
Which response includes all the following statements that are true and no false statements.
Redox reactions are characterized by the actual or formal transfer of electrons between chemical species most often with one species the reducing agent undergoing oxidation losing electrons while.
Sample Learning Goals Use the number of protons neutrons and electrons to draw a model of the atom identify.
The next step is to determine how many d-electrons the Fe 3 ion has.
The outer electrons in the valence band are loosely attached to the atomWhen an electron gets excited due to electromotive force or thermal effect it moves from its valence band to the conduction band.
Locate the transition metal on the periodic table and make note of the group number.
The rule is to count all of irons valence electrons as d-electrons.
The properties of a solid can usually be predicted from the valence and bonding preferences of its constituent atoms.
For example atoms in Groups 1 and 2 have 1 and 2 valence electrons respectively.
Use iron as an example a transitional metal with the symbol Fe atomic number 26 located at period 4 group 8.
Hydrogen-bonded solids such as ice make up another category that is important in a few crystals.
Valency is a measure of the ability of an atom to bond with other atoms.
It is incorporated in the mineral ferrihydrite FeOOH 8 FeOH 2 PO 4 which is attached to the inner wall of the sphere.
The direction of an arrow in this diagram indicates the spin of an electron either spin up or spin down.
For example the atoms of the elements in Group 1 of the periodic table all have one valence electron the atoms of the elements in Group 2 have two valence electrons and so on until Group 18 whose elements contain eight valence electrons is reached.
To release iron when the body needs it the iron must be changed from the FeIII to the FeII oxidation state.
Elements in their standard state also have the same number of electrons.
Each element has a unique atomic number.
Valence electrons are the electrons present in the outermost shell of an atom.
Then play a game to test your ideas.
Build an atom out of protons neutrons and electrons and see how the element charge and mass change.
Thus phosphorus can react with fluorine to form both PF 3 and PF 5.
The formalism has been incorporated into the two major models used to describe coordination complexes.
Group 8 - 3 charge d 5 or 3d 5 8 - 3 5.
Carbon has an outer shell consisting of 4 valence electrons.
Inside the sphere iron is stored in the FeIII oxidation state.
1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2.
Ferritin has the shape of a hollow sphere.
Calcium a chemical element with the symbol Ca and atomic number 20 is a reactive metal that can form a dark oxide-nitrite layer when exposed to the atmosphere.
By strict definition most transitional metals have two valence electrons but may have a larger range of apparent valence electrons.
By difference iron must be Fe 3 because the charges 3 61- must add up to the overall -3 charge on the complex.
The substance of the electrical conductor atom must have no energy gap between its valence band and conduction band.
The next orbital holds up to 8 electrons.
Electrons will occupy the most stable position first.
A 0 b 1 c 2 d 4 e 6 18.
In the case of iron the highest.
It is the third most abundant metal on the earths surface after iron and aluminum.
The d electron count is an effective way to understand the geometry and reactivity of transition metal complexes.
You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table.
The inner orbital holds up to 2 electrons.
This means it can either add 4 electrons to gain a full outer shell or lose 4 electrons to get rid of its outer shell.
Valence electrons are also the determining factor in some physical properties of the elements.
Phosphorus however has empty 3d atomic orbitals that can be used to expand the valence shell to hold 10 or more electrons.
Crystal Field Theory Consider the complex ion MnOH 2 6 2 with 5 unpaired electrons.
When it forms an Fe 3 ion it loses the 4s electrons and one of the 3d electrons.

High School Chemistry Electron Configurations Of Main Group Elements Wikibooks Open Books For An Open World

Electron Configuration Electron Shell Valence Electron Carbon Carbon Chemical Element Angle Png Pngegg
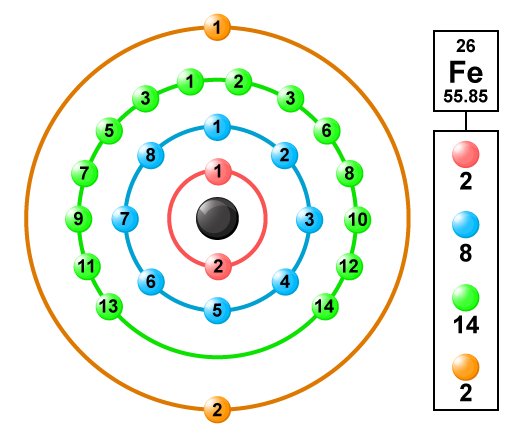
The Atomic Number Of Iron Is 26 Its Electronic Configuration 2 8 8 8 Then How Its Valency Is 2 And3 Chemistry Topperlearning Com Aaqtczee

Chemistry291 Hand Note 5 Steps How Many Valence Electrons Does Sodium Electrons Electron Configuration Chemical Equation
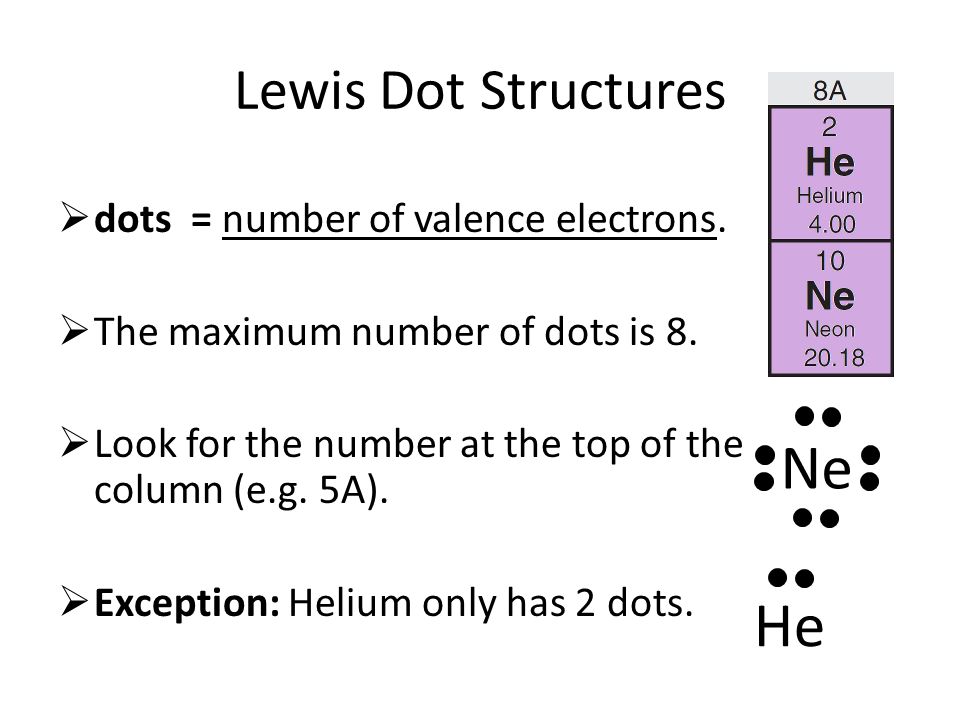







No comments:
Post a Comment