
1 In Bohr S Model Of H Atom The Kinetic Energy Of The Electron In Any Orbit Depends On The Principal Quantum Number N As 1 K 2 Konz 3 Koc 1 N 4 K

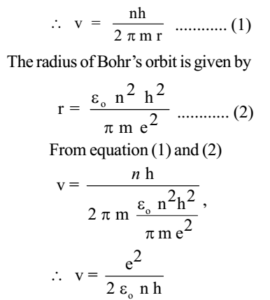
1 The First Bohr S Radius For Electron In Hydrogen Atom In The Ground State 2 The Ground Energy Level In Hydrogen Atom Ppt Video Online Download

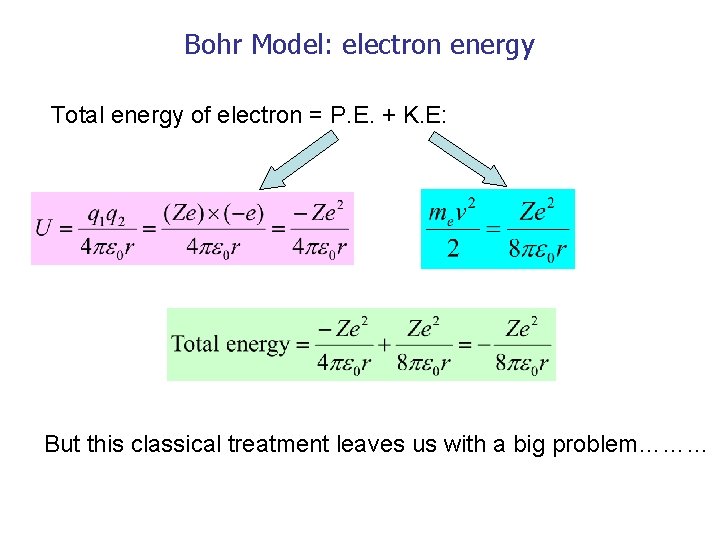
In The Bohr Model Of The Hydrogen Atom The Ratio Of The Kinetic Energy To The Total Energy Of The Electron In A Quantum State N Is
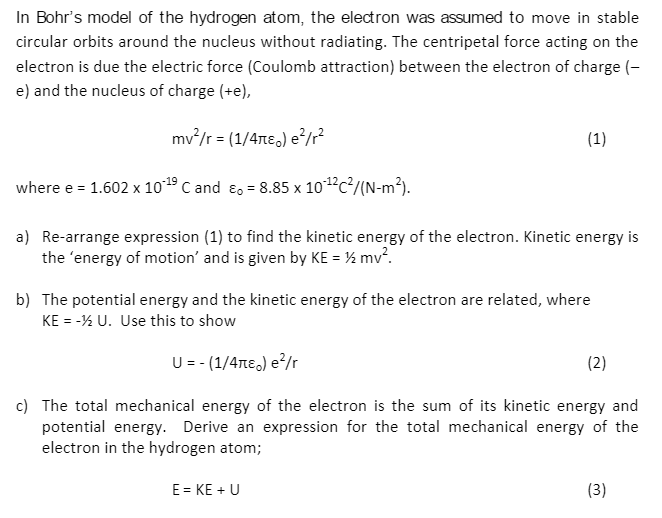
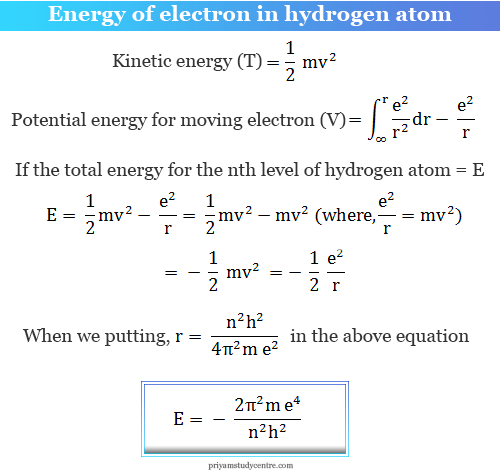
Using Bohr S Postulates Obtain The Expression For I Kinetic Energy And Ii Potential Energy Of The Electron In Stationary State Of Hydrogen Atom Sarthaks Econnect Largest Online Education Community

The Kinetic Energy Of An Electron In The Second Bohr Orbit Of S H Atom Is A0 Is Bohr Radius Chemistry Topperlearning Com Ipi5hvzz

In The Bohr S Model Of The Hydrogen Atom The Ratio Of The Kinetic Energy To The Total Energy Of The Electron In A Quantum State N Is


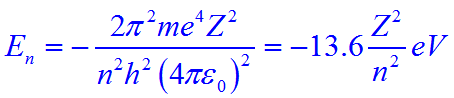

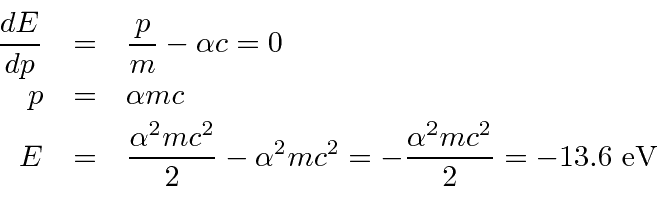





No comments:
Post a Comment