
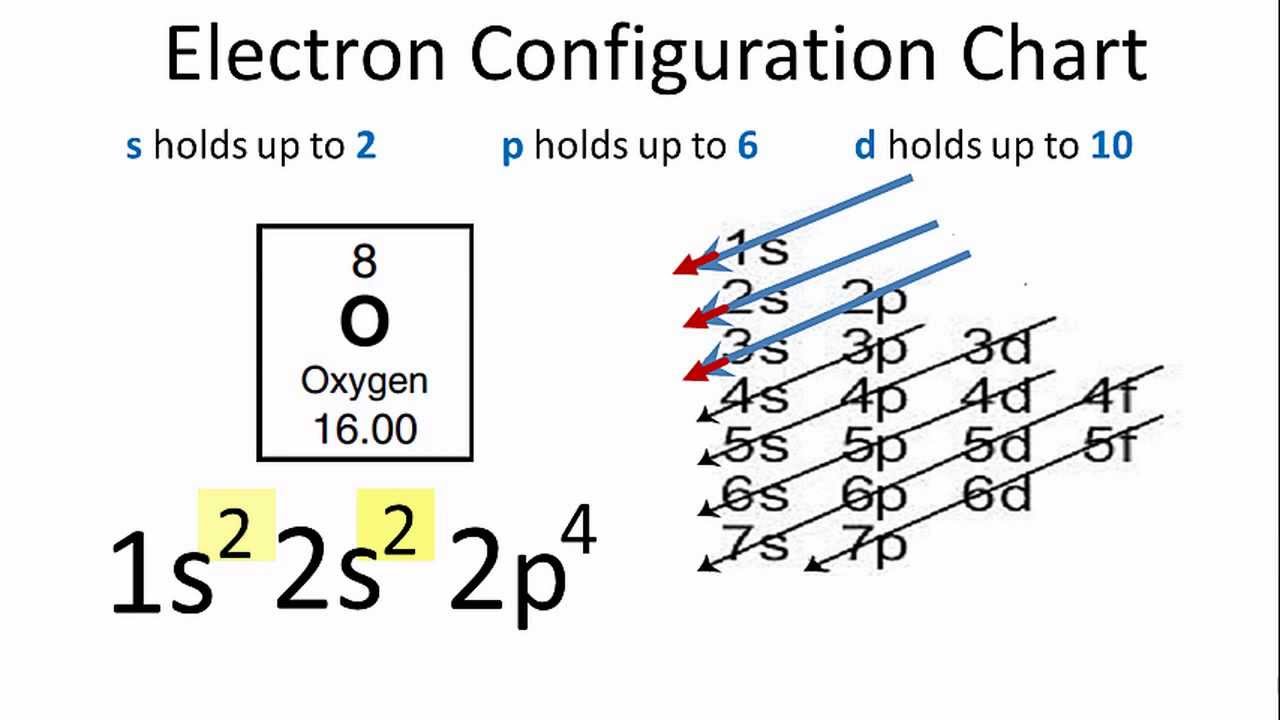
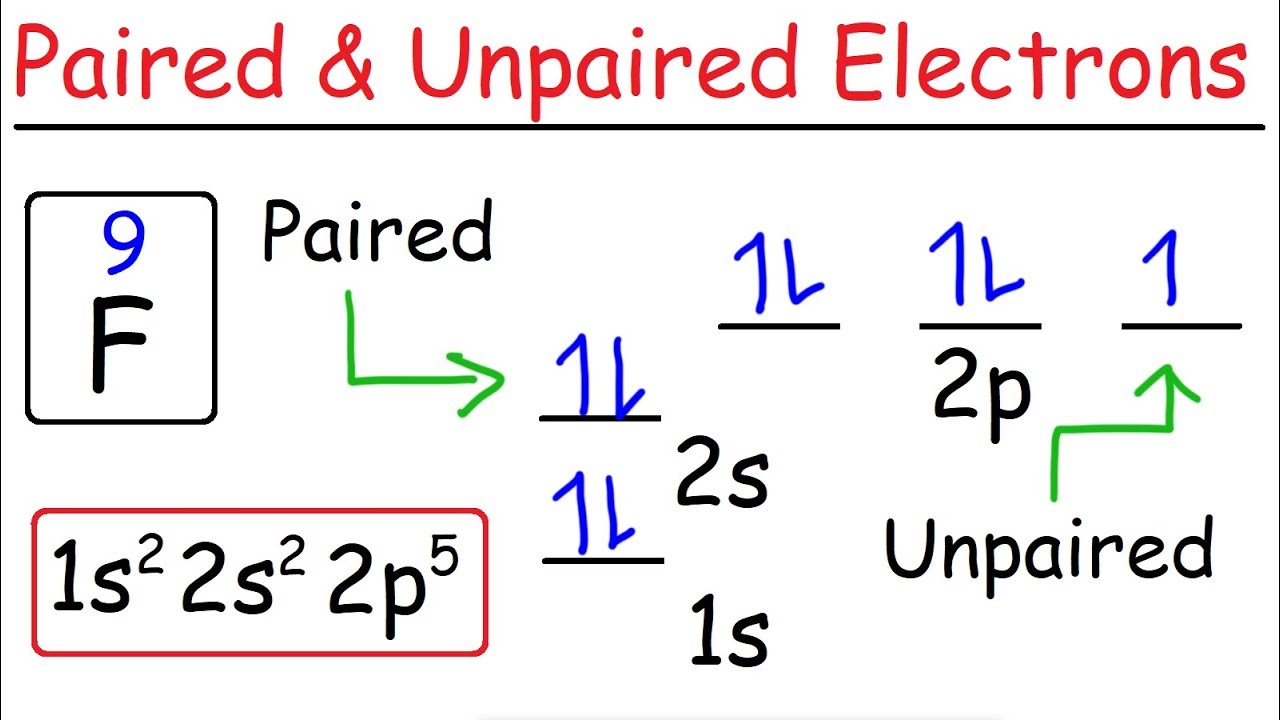
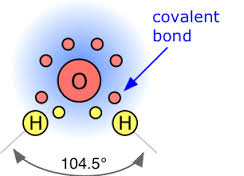
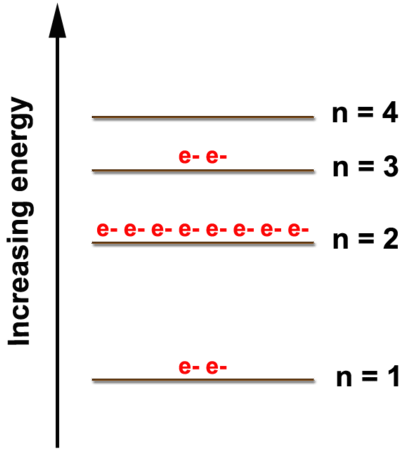
An Atom Of Oxygen Has Six Valence Electrons In Nature Oxygen Is A Diatomic Molecule And Is Usually Found In The Form O2 Why Would One Atom Of Oxygen Want To Bond

Valence Electrons Worksheet Worksheet C19 Valence Electrons And Electron Reading Graphs Text Features Worksheet Complex Sentences Worksheets

Figure 2 A A Star Core Contains Oxygen Neon And Magnesium Once The Core Density Becomes High Enough B Magne Neutron Star Electrons Supernova Explosion















No comments:
Post a Comment