While inner electrons those not in the valence shell typically dont participate in chemical bonding and reactions valence electrons can be gained lost or shared to form chemical bonds. Number of electrons it has to lose to become stable 6.

Finding The Number Of Valence Electrons For An Element Wayne Breslyn Simply Describes The Number Of Va High School Chemistry Secondary Science Teaching Science

How To Find Valence Electrons And Total Electrons Youtube Chemistry Lessons Teaching Chemistry Chemistry Help
At normal temperature and pressure two atoms of nitrogen bind together to form colorless and odorless dinitrogen N 2 gas.

How many valence electrons.
How Many Valence Electrons Does Nitrogen N Have.
Meanwhile the number of valence electrons present also helps us determine a specific elements chemical properties such as its valence or valency the formation of bonds with other elements.
Look it up now.
Whether it is electronegative or electropositive in nature or they indicate the bond order of a chemical compound the number of bonds that can be formed between two atoms.
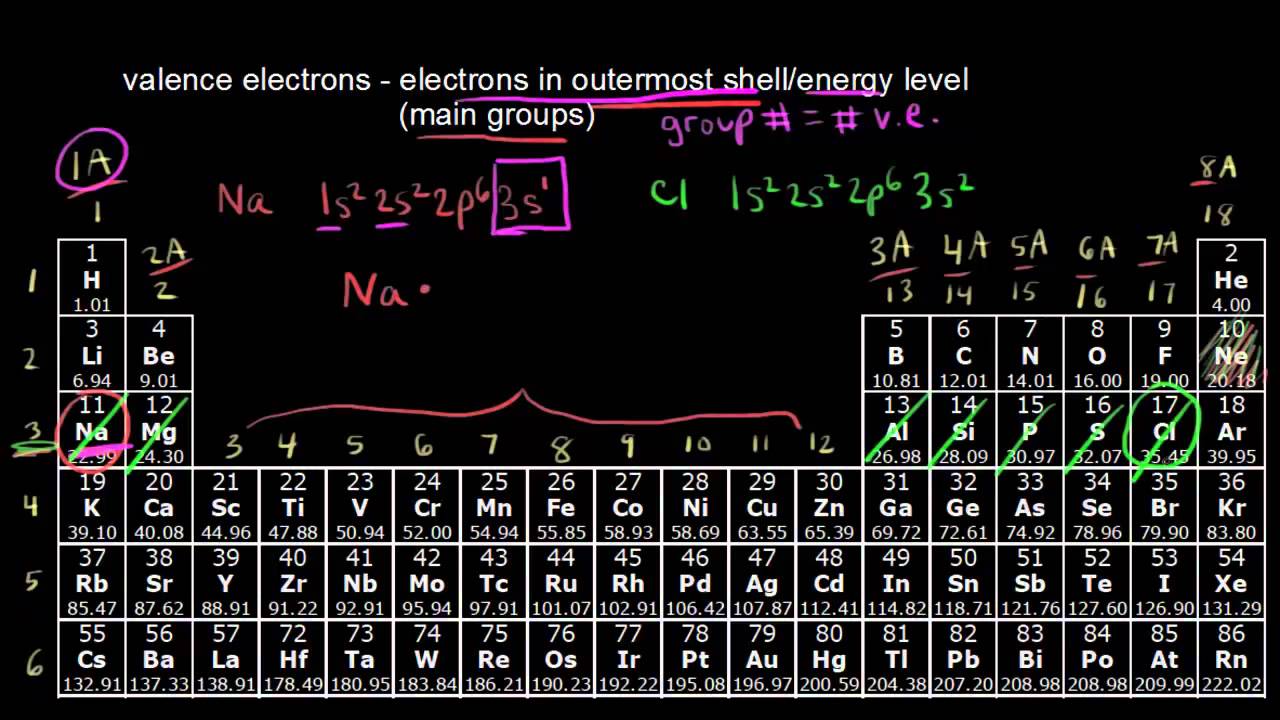
The column an element is in on the periodic table will indicate how many valence electrons it has and for now when we count across columns we.
For example Ca 2 has zero valence electrons.
Powered by FlexBook textbook Platform CK-12 Foundation 2021.
An electron is a stable negatively charged component of an atomElectrons exist outside of and surrounding the atom nucleus.
The number of valence electrons in an atom governs its bonding behavior.
Accordingly valence electrons directly influence how elements behave in a chemical reaction.
That means an atomic number of 8 oxygen has 8 protons and 8 electrons.
Imagine the atom is a set of circles with a dot in the.
In chemistry and atomic physics an electron shell may be thought of as an orbit followed by electrons around an atoms nucleusThe closest shell to the nucleus is called the 1 shell also called the K shell followed by the 2 shell or L shell then the 3 shell or M shell and so on farther and farther from the nucleusThe shells correspond to the principal quantum numbers n.
How Many Valence Electrons Does Hydrogen H Have.
Ca normally has 2 valence electrons but with a 2 charge it is missing two electrons.
Nitrogen a chemical element with the symbol Si and atomic number 14 is a colorless liquid gas or solid.
There are 7 valence electrons because the highest energy level 3 has 7 total electrons 5 plus 2 is 7.
Number of shells holding the maximum number of electrons 19 G.
A positively charged atom has fewer electrons than the normal valence number.
You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table.
Each electron carries one unit of negative charge 1602 x 10-19 coulomb and has a small mass as compared with that of a neutron or protonElectrons are much less massive than protons or neutrons.
The most reactive kind of metallic element is an alkali metal of group 1 eg sodium or potassium.
Lets draw it out as a simple diagram.
This is because such an atom has only a single valence electron.
The mass of an electron is 910938 x 10-31 kg.
For example atoms in Groups 1 and 2 have 1 and 2 valence electrons respectively.
If the number is larger than 10 subtract 10 so you get two valence electrons.
Each square on the periodic table contains the letter symbol for an element printed directly below the atomic number of the element.
Number of protons 3.
The atomic number is how many protons and electrons the atom has.
The number of valence electrons of an element can be determined by the periodic table group vertical column in which the element is categorized.
They have the same number of electron shells.
A way to find valence electrons without the periodic table is using the atomic number and drawing a diagram.
If you look at the periodic table and at the period numbers that is the number of valence electrons.
About 75 of all.
É number of shells 2.
Total number of electrons 4.
Valence electrons are those electrons that reside in the outermost shell surrounding an atomic nucleus.
Knowing how to find the number of valence electrons in a particular atom is an important skill for chemists because this information determines the kinds of chemical bonds that it can form and therefore the elements reactivity.
It also gives us an idea of how readily the atoms can form bonds the number of unpaired electrons and how many atoms can take part.
Valence electrons are the electrons contained in the outermost shell.
Hydrogen a chemical element with the symbol H and atomic number 1 is the lightest element in the periodic table with a standard atomic weight of 1008.
Locate the desired element on the periodic table.
They have the same number of electrons.
So it can be understood that the number of valence electrons main group number - chargecharged atoms.
3 num r of valence electr ns 5.
Finding Valence Electrons for All Elements Except Transition Metals.
Therefore elements whose atoms can have the same number of valence electrons are grouped together in the periodic table of the elements.
With the exception of groups 312 the transition metals the units digit of the group number identifies how many valence electrons are associated with a neutral atom of an element listed under.
In chemistry valence electrons are the electrons that are located in the outermost electron shell of an element.
Valence electrons are the electrons present in the outermost shell of an atom.
Valency of Nitrogen by Richard-January 28 2021 0.
Valence electrons are the highest energy electrons in an atom and are therefore the most reactive.
Valency of H H by Richard-January 14 2021 0.
Oxygen is in the 16th period.
For calcium which has an atomic number of 20 and therefore 20 electrons find calcium on.
None of the above 1.
It is one of the most abundant chemical elements found in the universe.
Valence electrons are of crucial importance because they lend deep insight into an elements chemical properties.

Chemistry291 Hand Note 5 Steps Electron Configuration For Potassium Or O In 2021 Electron Configuration Configuration Teaching Chemistry

Valence Electrons Coloring Activity Chemistry Science Pdf Printable From Laurelsusanstudio On Teache Chemistry Activities Chemistry Classroom Science Chemistry

Valence Electrons And The Periodic Table Tyler Dewitt Video November 10 2017 Chemistry Electrons Periodic Table

Valence Electron Determination Practice Chemistry Homework Worksheet Homework Worksheets Chemistry Chemistry Notes

What Are Valence Electrons And What Is The Atomic Structure Video Teaching Chemistry Science Lessons Physical Science Lessons

Electron Configuration Part 2 Chemistry Refresher For Organic Chemistry Students To Help You Identify The Electron Configuration Organic Chemistry Chemistry

Science Coverage Valency Of Cesium How Many Valence Electrons Doe In 2021 Electron Configuration Electrons Noble Gas

Chemistry291 Hand Note 5 Steps How Many Valence Electrons Does Iodine I Electrons Electron Configuration Chemical Equation

How Many Valence Electrons Does Carbon Have Perfect Atom Electrons Electron Configuration Business Plan Template














No comments:
Post a Comment